- Home >
- Publications » Recent Publications
Hysteretic Control of Near-infrared Transparency Using a Liquescent Radical Cation
Shuichi Suzuki, Daiki Yamaguchi, Yoshiaki Uchida, Takeshi Naota,
Angew. Chem. Int. Ed., 60, 8284-8288 (2021).
A liquescent dihydrophenazine radical cation showed drastic changes in near‐infrared (near‐IR) transparency and opaqueness through hysteretic phase transitions with no measurable degradation of the compound even under aerated conditions. During the heating and slow cooling process, its electronic and magnetic properties were altered clearly and repeatedly changed between solid and liquid states. The liquid state was transparent to near‐IR light (940 nm), but the solid state was opaque, despite both samples exhibiting a similar green color under room light. Additionally, the liquid state was changed to a glass state under a fast cooling process. UV/Vis/near‐IR and electron spin‐resonance spectroscopy revealed that these drastic changes were attributable to the dynamic dissociation and association of a pi‐dimer structure for the radical cation accompanying with the solid-liquid phase transitions even under the neat conditions.
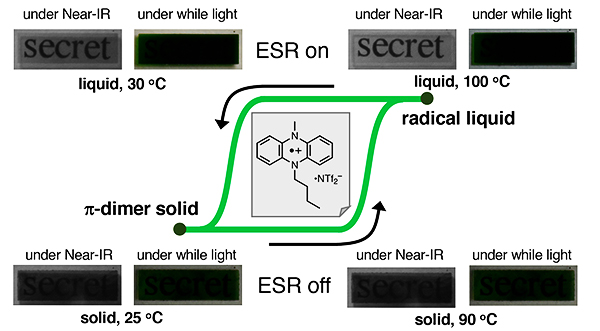
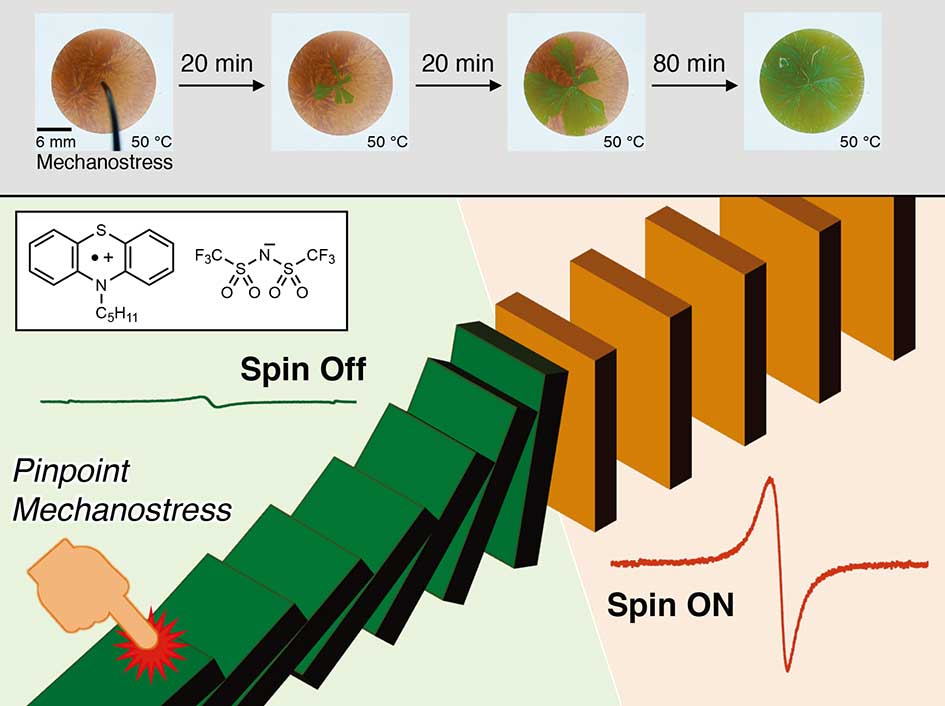
Strategy for Stimuli-Induced Spin Control Using a Liquescent Radical Cation
Shuichi Suzuki, Ryochi Maya, Yoshiaki Uchida, Takeshi Naota,
ACS Omega, 4, 10031-10035 (2019).
A liquescent salt based on an N-pentylphenothiazine radical cation exhibited a unique phase transition from a paramagnetic orange solid to a diamagnetic green solid induced by brief, weak, and pinpoint mechanostress. ESR and UV/Vis/NIR spectra showed that this unprecedented solid-state spin controllability was attributable to mechanostress-triggered sequential association even under neat conditions.
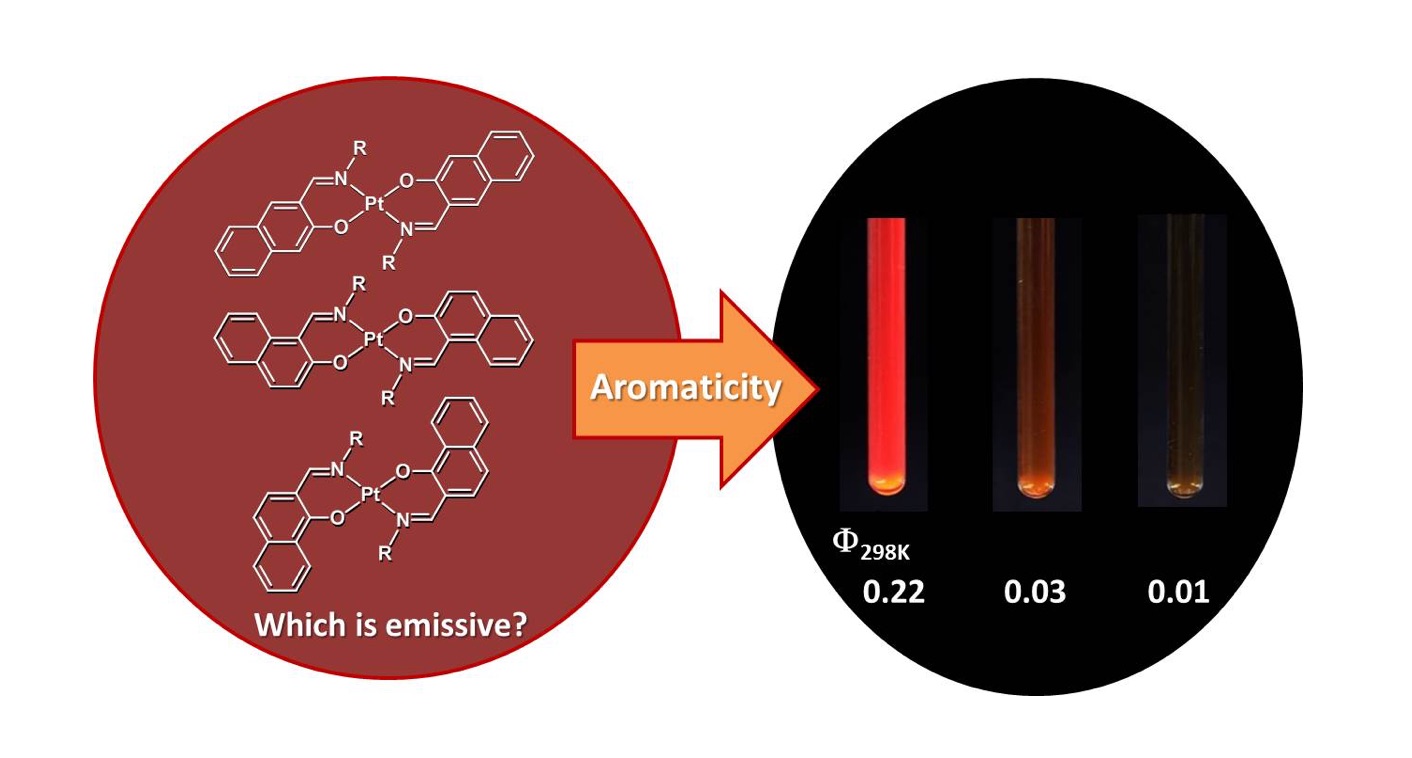
Heat-Resistant Properties in the Phosphorescence of trans-Bis[β-(iminomethyl)aryloxy]platinum(II) Complexes: Effect of Aromaticity on d–π Conjugation Platforms
Ryo Inoue, Masaya Naito, Masahiro Ehara, Takeshi Naota ,
Chem. Eur. J., 25, 3650-3661 (2019).
The heat‐resistant properties towards thermal emission quenching of trans‐bis[(β‐iminomethyl)aryloxy]platinum(II) complexes bearing 3‐iminomethyl‐2‐naphtholato‐, 1‐iminomethyl‐2‐naphtholato‐, 2‐iminomethyl‐1‐naphtholato‐, and 2‐iminomethyl‐1‐phenolato moieties, and a mechanistic rationale of these properties, are reported. 3‐Iminomethyl‐2‐naphtholato platinum with N,N′‐dipentyl groups, exhibits intense red emission in 2‐MeTHF at 298 K, whereas the other analogues are less or non‐emissive under the same measurement conditions. We investigated the emission decay and thermal‐deactivation processes using DFT, TDDFT, and double‐hybrid density functional theory (DHDF) calculations of these complexes, and discuss the results with a focus on the energy levels, molecular structures, and electronic configurations in the triplet excited states. The energy differences between the (3MLCT) state and minimum‐energy crossing point between the lowest triplet state and singlet ground state (MECP) increase, that consistent with the experimental results for the heat‐resistant properties of these complexes. The origin of the present structure dependence of the 3MLCT–MECP energy gap is ascribed to the ease or difficulty of the high‐lying dσ* orbital participating in the MECP upon thermal structural distortion. The structure dependence in energy gaps between the π* and dσ* orbitals, which is key for facilitating the thermal deactivation process, is rationally correlated with the extent of aromaticity on the coordination platforms.
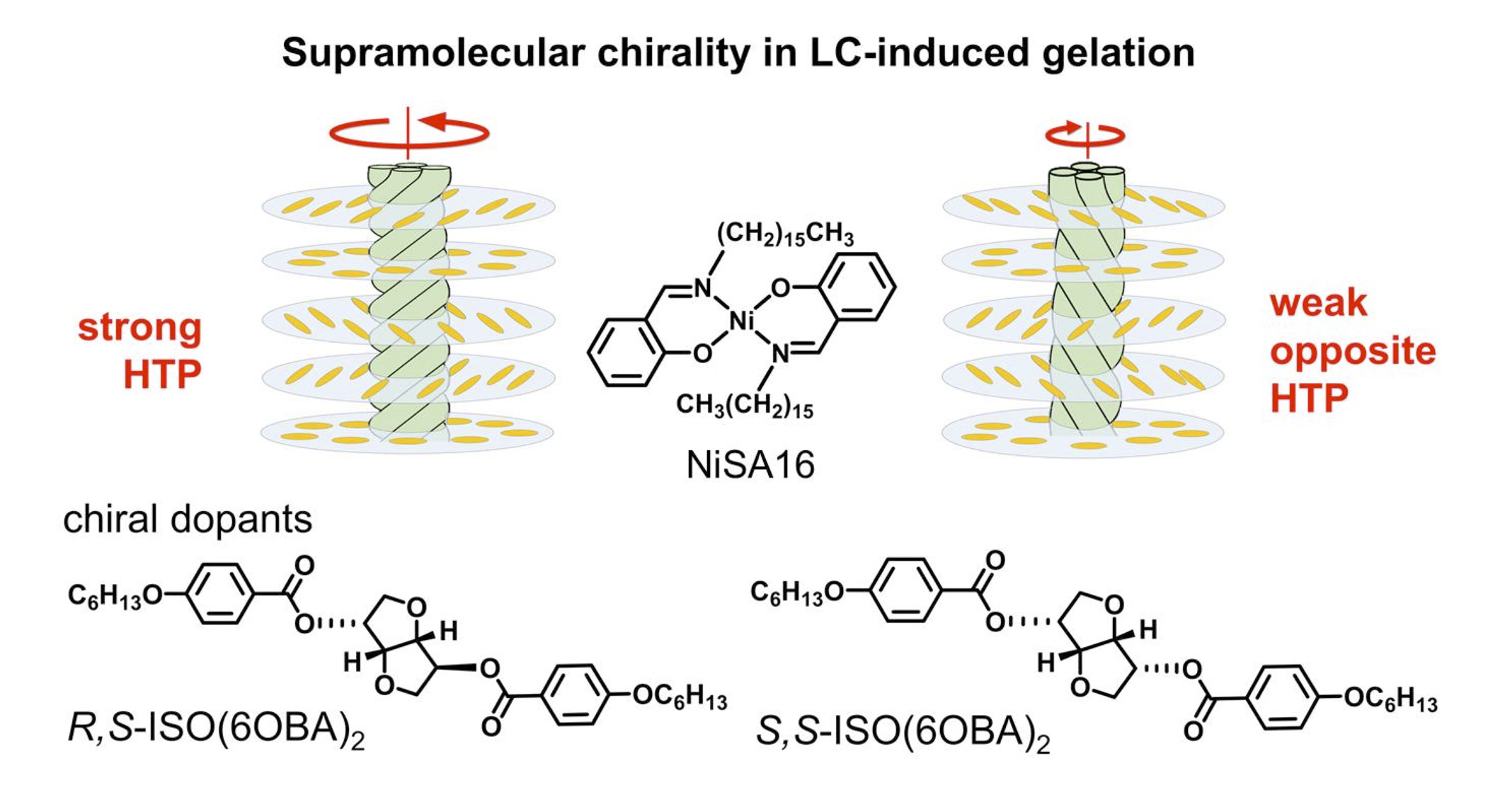
Helicity Control of Supramolecular Gel Fibers Consisting of an Achiral NiII Complex in a Chiral Nematic Solvent
Takatoshi Maeda, Yuuki Kuwajima, Takuya Akita, Yosuke Iwai, Naruyoshi Komiya, Yoshiaki Uchida, Takeshi Naota,
Chem. Eur. J., 24, 12546–12554(2018).
The supramolecular chirality of aggregates consisting of an achiral NiII complex can be generated and controlled precisely in chiral nematic liquid crystalline (LC) solvent, while the complex naturally forms achiral gel fibers in achiral nematic LC solvents and crystals in non‐mesogenic solvents. Direction and intensity in the helicity of the gel fibers in the LC gel state can be adjusted according to the helical twisting nature and concentration of the chiral dopants.
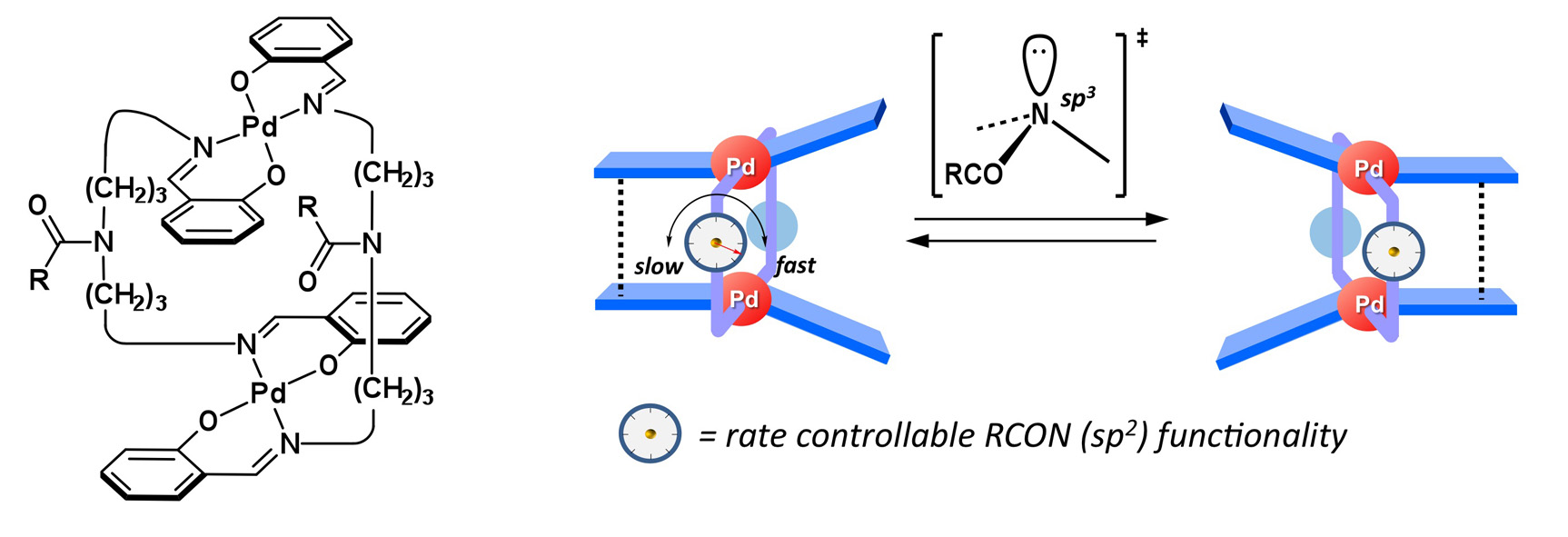
Single-point Remote Control of Flapping Motion in Clothespin-shaped Bimetallic Pd Complexes Based on N(sp2)―N(sp3) Interconversion on Amide Functionalities
Ryo Inoue, Soichiro Kawamorita, Takeshi Naota,
Chem. Eur. J., 22, 5712–5726(2016).
The unique flapping mobility of clothespin shaped complexes in the solution state can be controlled remotely according to the electronic and steric factors of amide functionalities on the 4-azaheptamethylene linkers. Structural and kinetic parameters obtained based on XRD analyses, DFT calculations and 2D EXSY experiments revealed that nitrogen pyramidalization on the azalinkers is a critical step for the multi-step conformational transfers during the motion.
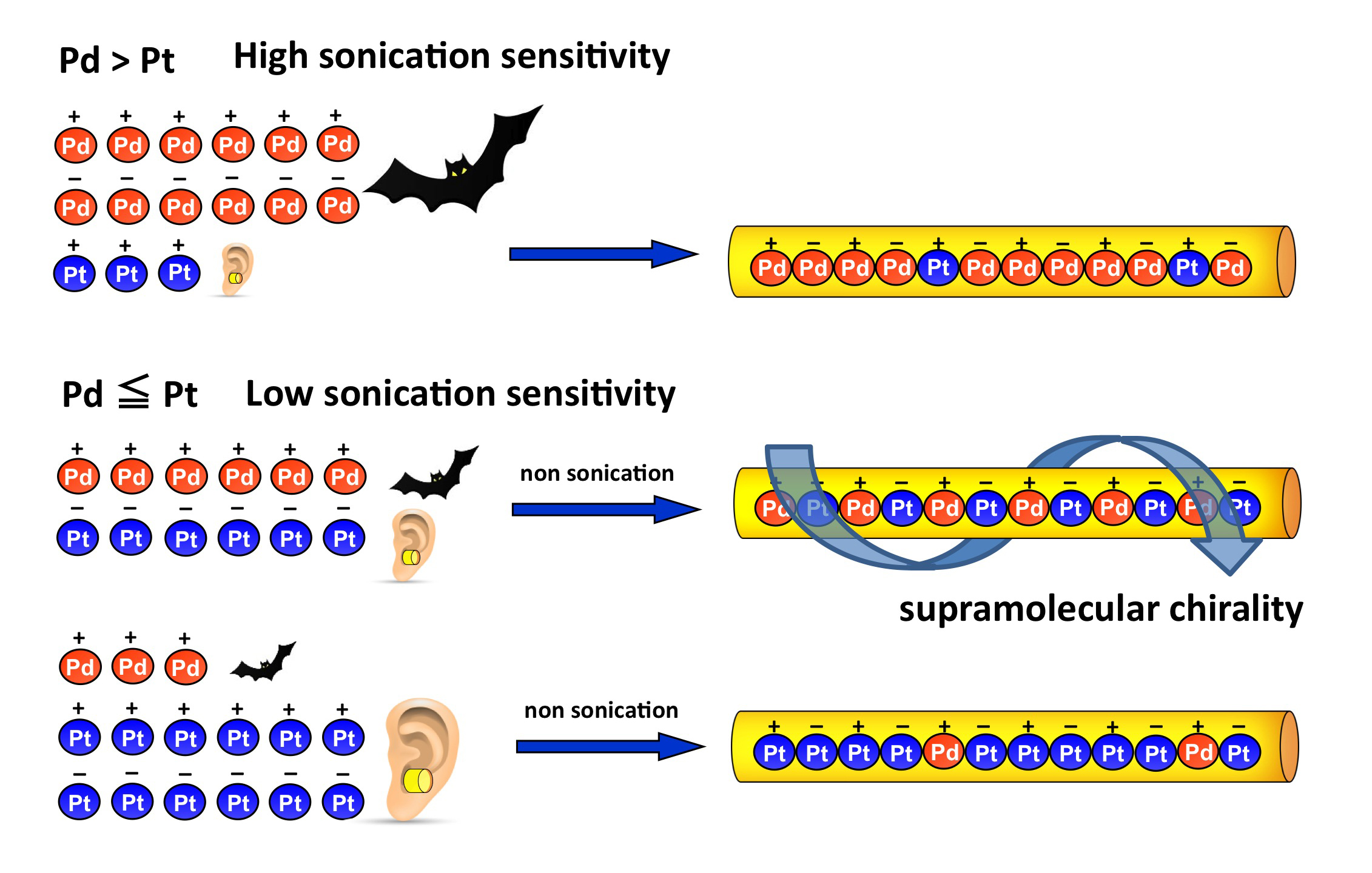
Control of Metal Arrays Based on Heterometallics Masquerading in Heterochiral Aggregations of Chiral Clothespin-shaped Complexes
M. Naito, R. Inoue, M. Iida, Y. Kuwajima, S. Kawamorita, N. Komiya and Takeshi Naota,
Chem. Eur. J., 21, 12927-12939 (2015).
Heterometallic arrays, ultrasound responsiveness and supramolecular chirality in molecular aggregation can be precisely controlled by the gelation of organic liquids containing the chiral, clothespin-shaped trans-bis(salicylaldiminato) d8 transition metal complexes.
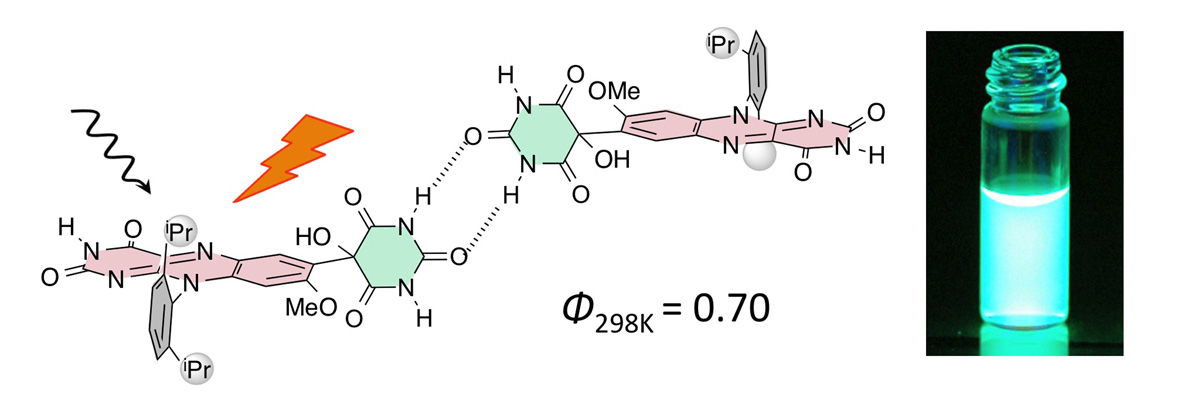
Highly Fluorescent Flavins: A Molecular Design Rationale for Quenching Protection Based on Repulsive and Attractive Control of Molecular Alignment
H. Suzuki, R. Inoue, S. Kawamorita, N. Komiya, Y. Imada and T. Naota,
Chem. Eur. J., 21, 9171-9178 (2015).
Unprecedented intense fluorescent emission is observed for a variety of flavin compounds 3 and 4 bearing a perpendicular cyclic imide moiety at the C(7)-position of an isoalloxazine platform. A series of alloxan-substituted flavin 3 was prepared selectively by reduction of the corresponding N-aryl-2-nitro-5-alkoxyanilines 6 with zinc dust and subsequent reaction with alloxan monohydrate (7) in the presence of boric acid. Analogues 4 bearing the oxazolidine-2,4-dione functionality were obtained upon methylation of 3 with methyl iodide and subsequent rearrangement in the presence of an inorganic base. Compounds 3 and 4 exhibit intense white-green fluorescent emission in the solution state under UV excitation at 298 K, with emission efficiencies (Φ298K) of over 0.55 in CH3CN, which are higher than the values for all the reported flavin compouds under the similar conditions. The highest Φ298K value of 0.70 was obtained in CH3CN for isoalloxazine 3a bearing C(7)-alloxan and N(10)-2,6-diisopropylphenyl functionalities. The temperature dependence of the emission intensities for 3 indicate that the high emission properties at 298 K are attributable to the specifically high heat resistant properties towards emission decay with increasing temperature. Mechanistic studies including X-ray diffraction analysis of 3b revealed that high emission properties and heat resistance of 3 are due to a synergetic effect of the functionalities from the association factor of the C(7)-alloxan unit with the repulsive factor of the perpendicular bulky substituents at the C(7)- and N(10)-positions.
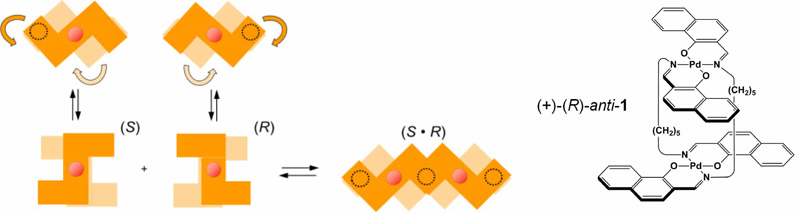
Binuclear trans-Bis(beta-iminoaryloxy)palladium(II) Complexes Doubly Linked with Pentamethylene Spacers: Structure-dependent Flapping Motion and Heterochiral Association Behavior of the Clothespin-shaped Molecules,
M. Naito, H. Souda, H. Koori, N. Komiya, T. Naota,
Chem. Eur. J., 20, 6991-7000 (2014).
The synthesis, structure, and solution-state behavior of clothespin-shaped binuclear trans-bis(beta-iminoaryloxy)-palladium(II) complexes doubly linked with pentamethylene spacers are described. Achiral syn and racemic anti isomers of complexes 1?3 were prepared by treating Pd(OAc)2 with the corresponding N,N’-bis(beta-hydroxyarylmethylene)-1,5-pentane-diamine, and subsequent chromatographic separation. Optically pure (100% ee) complexes, (+)-anti-1, (+)-anti-2 and (+)-anti-3 were obtained from the racemic mixture using a preparative HPLC system with a chiral column. The trans-coordination and clothespin-shaped structures with syn- and anti-conformations of these complexes have been unequivocally established from X-ray diffraction studies. 1H NMR analysis showed that (±)-anti-1, (±)-anti-2, syn-2, and (±)-anti-3 make a flapping motion by consecutive stacking association/ dissociation between cofacial coordination planes in toluene-d8, while syn-1 and syn-3 is static under the same conditions. The activation parameters for the flapping motion (DH‡ and DS‡) were determined from variable temperature NMR analyses to be 50.4 kJ mol-1 and 60.1 J mol-1 K-1 for (±)-anti-1, 31.0 kJ mol-1 and ?22.7 J mol-1 K-1 for (±)-anti-2, 29.6 kJ mol-1 and ?57.7 J mol-1 K-1 for syn-2, and 35.0 kJ mol-1 and 0.5 J mol-1 K-1 for (±)-anti-3, respectively. The molecular structure and kinetic parameters demonstrate that all anti complexes flap with twisting motion in toluene-d8, where (±)-anti-1 bearing dilated Z-shaped blades move more dynamically than I-shaped (±)-anti-2 and smaller (±)-anti-3. Highly symmetric syn-2 performs a much more static flapping motion, i.e., in a see-saw-like manner. In CDCl3, (±)-anti-1 exhibits an extraordinarily upfield shift of the 1H NMR signals with increasing concentration, while solutions of (+)-anti-1 and other syn/anti-analogues
Solid-State Phosphorescence of trans-Bis(salicylaldiminato)platinum(II)
Complexes Bearing Long Alkyl Chains: Morphology Control towards Intense
Emission,
N. Komiya, N. Itami, T. Naota,
Chem. Eur. J., 19, 9497-9505 (2013).
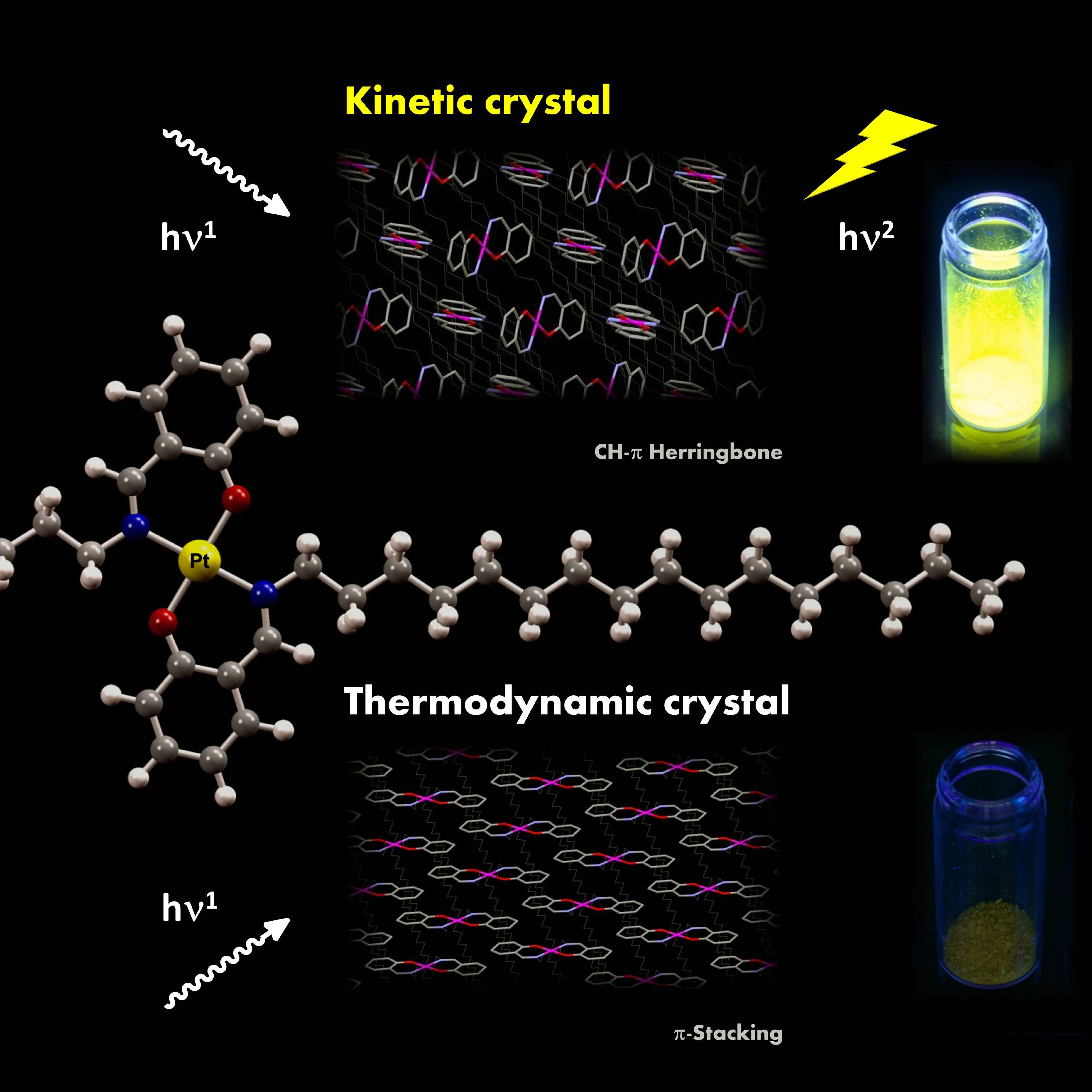
Morphology control for intense solid-state phosphorescence of non-emissive, but potentially emissive crystals of platinum complexes and the mechanistic rationale are described. A series of trans-bis(salicylaldiminato)platinum(II) complexes bearing linear alkyl chains (1a: n=5; 1b: n=8; 1c: n=12; 1d: n=14; 1e: n=16; 1f: n= 18) was synthesized and the solid-state emission properties were examined by using crystals/aggregates prepared under various precipitation conditions. Crystals of 1e, prepared using “kinetic” conditions including rapid cooling, high concentrations, and poor solvents, emit intensive yellow phosphorescence (λmax = 545 nm) under UV irradiation at 298 K with an absolute quantum efficiency of 0.36, whereas all the crystals of 1a?1f prepared using “thermodynamic” conditions including slow cooling, low concentrations, and good solvents were either non- or less emissive with Φ298K values of 0.12 (1a), 0.11 (1b), 0.10 (1c), 0.07 (1d), 0.02 (1e), and 0.02 (1f) under the same measurement conditions. The amorphous solid 1e, prepared by rapid cooling and freeze-drying, was also non-emissive (Φ298K = 0.02, 0.02). Temperature-dependent emission spectra showed that the kinetic crystals of 1e exhibit high heat-resistance towards emission decay with increasing temperature, whereas the amorphous solid 1e is entirely heat-quenchable. This is a rare example of the change from a non-emissive crystal into a highly emissive crystal by morphology control through crystal engineering. Emission spectra and powder X-ray diffraction (XRD) patterns of the emissive, kinetic crystals of 1e are clearly distinct from those of the less emissive, thermodynamic crystals of 1a?1f. Single-crystal XRD unequivocally establishes that the thermodynamic crystals of 1d have a multilayered lamellar structure supported by highly regulated, consecutive p-stacking interactions between imine moieties, whereas the kinetic crystals of 1e have a face-to-edge lamellar structure with less stacking. These results lead to the conclusion that 1) morphology control of long-chained complexes exclusively generates a metastable herring- bone-based lamellar packing motif that exhibits intense emission and high heat-resistance, while 2) a thermodynamically stable, highly regulated, consecutive stacking motif is unfavorable for solid-state emission.
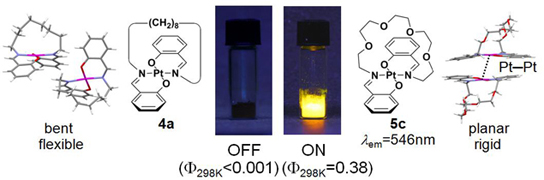
Vaulted trans-Bis(salicylaldiminato)platinum(II) Crystals: Heat-Resistant,
Chromatically Sensitive Platforms for Solid-State Phosphorescence at Ambient Temperature,
N. Komiya, M. Okada, K. Fukumoto, K. Kaneta, A. Yoshida, T. Naota,
Chem. Eur. J., 19, 4798-4811 (2013).
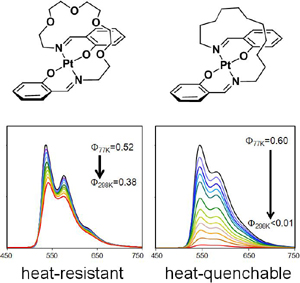
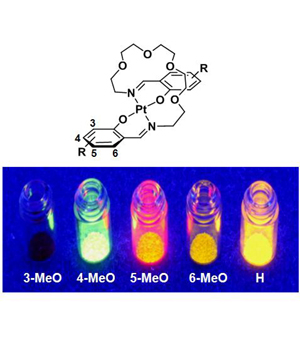
The synthesis, structure, and solid-state emission of vaulted trans-bis(salicylaldiminato)platinum(II)complexes are described. A series of polymethylene (1: n=8; 2: n=9; 3: n=10; 4: n=11; 5: n=12; 6: n=13) and polyoxyethylene (7: m=2; 8: m=3; 9: m=4) vaulted complexes (R=H (a), 3-MeO (b),4-MeO (c), 5-MeO (d), 6-MeO (e), 4-CF3O (f),5-CF3O (g)) was prepared by treating [PtCl2(CH3CN)2] with thecorresponding N,N′-bis(salicylidene)-1,ω-alkanediamines. The trans coordination, vaulted structures, and the crystal packing of 1–9 have been unequivocally established from X-ray diffraction studies.Unpredictable, structure-dependent phosphorescent emission has been observed for crystals of the complexes under UV excitation at ambient temperature,whereas these complexes are entirely nonemissive in the solution state underthe same conditions. The long-linked complex crystals 4–6, 8, and 9 exhibit intense emission (Φ77K=0.22–0.88) at 77 K, whereas short-linked complexes 1–3 and 7 are non-or slightly emissive at the same temperature (Φ77K<0.01–0.18). At 298 K, some of the long-linked crystals, 4 a, 4 b, 5 c,5 e, 6 c, 6 e, and 9 b, completely lose theirhigh-emission properties with elevation of the temperature (Φ298K<0.01–0.02),whereas the other long-linked crystals, 5 a, 6 a, 9 a, and9 d, exhibit high heat resistance towards emission decay with increasing temperature (Φ298K=0.21–0.38). Chromogenic control of solid-state emission over the range of 98 nm can be performed simply by introducing MeO groups at different positions on the aromatic rings. Orange, yellow-green, red, and yellow emissions are observed in the glass and crystalline state upon 3-, 4-, 5-, and 6-MeO substitution, respectively, whereas those with CF3O substituents have orange emission, irrespective of the substitution position.DFT calculations (B3LYP/6-31G*, LanL2DZ) showed that such chromatic variationis ascribed to the position-specific influence of the substituents on the highest-occupied molecular orbital (HOMO) and lowest-unoccupied molecularorbital (LUMO) levels of the trans-bis(salicylaldiminato)platinum(II) platform. The solid-state emission and its heat resistance have been discussedon the basis of X-ray diffraction studies. The planarity of the trans-coordination sites is strongly correlated to the solid-state emission intensities of crystals 1–9 at lower temperatures. The specific heat-resistance properties shown exclusively by the 5 a, 6 a, 9 a, and 9 dcrystals are due to their strong three-dimensional hydrogen-bonding interactions and/or Pt⋅⋅⋅Pt contacts, whereasheat-quenchable crystals 4 a, 4 b, 5 c, 5 e, 6 c,6 e, and 9 b are poorly bound with limited interactions, such asnon-, one-, or two-dimensional hydrogen-bonding networks. These results lead tothe conclusion that Pt⋅⋅⋅Pt contacts are animportant factor in the heat resistance of solid-state phosphorescence at ambient temperature, although the role of Pt⋅⋅⋅Pt contacts can be substituted by only higher-ordered hydrogen-bonding fixation.
【Important Paper】
Ultrasound-Induced Emission Enhancement Based on Structure-Dependent
Homo- and Heterochiral Aggregations of Chiral Binuclear Platinum
Complexes,
N. Komiya, T. Muraoka, M. Iida, M. Miyanaga, K. Takahashi, and T. Naota,
J. Am. Chem. Soc., 133, 16054-16061 (2011).
Instant and precise control of phosphorescent emission can be performed by ultrasound-induced gelation of organic liquids with chiral, clothespin-shaped trans -bis(salicylaldiminato)Pt(II) complexes, anti - 1 . Nonemissive solutions of racemic, short-linked anti - 1a ( n = 5) and optically pure, long-linked anti - 1c ( n = 7) in organic liquids are transformed immediately into stable phosphorescent gels upon brief irradiation of low-power ultrasound. Emission from the gels can be controlled by sonication time, linker length, and optical activity of the complexes. Several experimental results indicated that structure-dependent homo- and heterochiral aggregations and ultrasound-control of the aggregate morphology are key factors for emission enhancement.
【Important Paper】【Osaka University 100 Papers Selections】
Highly Phosphorescent Crystals of Vaulted
trans-Bis(salicylaldiminato)platinum(II) Complexes,
N. Komiya, M. Okada, K. Fukumoto, D. Jomori, and T. Naota,
J. Am. Chem. Soc., 133, 6493-6496 (2011).
Unprecedented strong phosphorescent emission in the crystalline state is observed for a variety of vaulted trans-bis(salicylaldiminato)platinum(II) complexes which are newly synthesized as a third coordination mode of well-studied bis(salicylaldiminato) complexes. This Communication describes the dynamic photophysical properties of these complexes in the liquid and crystalline states and a mechanistic rationale for the strong emission in the crystalline state.
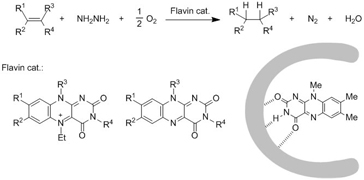
Aerobic Reduction of Olefins by In Situ Generation of Diimide with
Synthetic Flavin Catalysts,
Y. Imada, H. Iida, T. Kitagawa, and T. Naota, Chem. Eur. J., 17, 5908-5920 (2011).
A versatile reducing agent, diimide, can be generated efficiently by the aerobic oxidation of hydrazine with neutral and cationic synthetic flavin catalysts 1 and 2 . This technique provides a convenient and safe method for the aerobic reduction of olefins, which proceeds with 1?equiv of hydrazine under an atmosphere of O2 or air. The synthetic advantage over the conventional gas-based method has been illustrated through high hydrazine efficiency, easy and safe handling, and characteristic chemoselectivity. Vitamin B 2 derivative 6 acts as a highly practical, robust catalyst for this purpose because of its high availability and recyclability. Association complexes of 1?b with dendritic 2,5-bis(acylamino)pyridine 15 exhibit unprecedented catalytic activities, with the reduction of aromatic and hydroxy olefins proceeding significantly faster when a higher-generation dendrimer is used as a host pair for the association catalysts. Contrasting retardation is observed upon similar treatment of non-aromatic or non-hydroxy olefins with the dendrimer catalysts. Control experiments and kinetic studies revealed that these catalytic reactions include two independent, anaerobic and aerobic, processes for the generation of diimide from hydrazine. Positive and negative dendrimer effects on the catalytic reactions have been ascribed to the specific inclusion of hydrazine and olefinic substrates into the enzyme-like reaction cavities of the association complex catalysts.
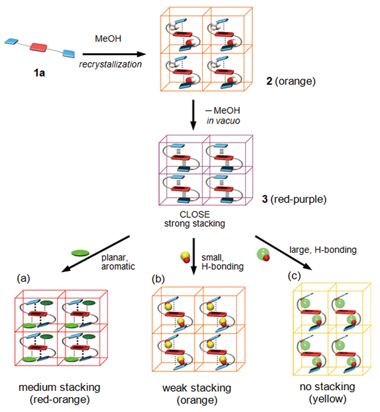
【Osaka University 100 Papers Selections】
Dynamic Vapochromic Behaviors of Organic Crystals Based on the Open Close Motions of S-Shaped Donor Acceptor Folding Units,
E. Takahashi, H. Takaya, and T. Naota, Chem. Eur. J., 16, 4793-4802 (2010).
The first vapochromic organic crystals are described with respect to their preparation, color change, adsorption/desorption properties, crystal structures, and color-change mechanism. Non-solvatochromic, 1,4,5,8-naphthalene-tetracarboxylic diimide (NDI) derivatives 1?a bearing two pyrrole imine (PI) tethers have been used as a motif for the crystal packing template. Red-purple vapochromic solid 3 was prepared by evacuation of orange crystals 2 (equivalent to 1?a ?2?MeOH), obtained by recrystallization of 1?a from MeOH. Solid 3 showed high-adsorption ability and unprecedented vapor-dependent color changes upon exposure to a variety of organic vapors, whereas light brown amorphous solid 1?a , did not show vapo- or solvatochromic behavior toward any organic solvent. The strong adsorption capability of 3 was confirmed by TGA experiments and adsorption/desorption isotherms. Analysis of the solid-state UV/Vis analysis revealed that the vapor-dependent color changes of 3 were owed to the specific interference of solvent vapors with its broad CT absorbance at λ =450?650?nm. Packing structures of 1?a in orange crystals 2 , red-purple solid 3 , and regenerated orange solid 2 were unequivocally established by single crystal and synchrotron powder X-ray diffraction, respectively. Molecular structures and arrays of 1?a in these materials indicated that 1)?unit 1?a had an S-shaped folded conformation in 2 and 3 by intramolecular donor?acceptor interactions between NDI and two PI units; 2)?inclusion of the guest vapor into the S-shaped template decreased the intramolecular PI-NDI interactions, accompanied by increasing intermolecular NDI-NDI and PI-PI interactions; and 3)?such flexible, open?close motions of the S-shaped template could be repeated during reversible adsorption/desorption processes without degradation of crystal packing. The adsorption properties and mechanism of molecular shape-dependent vapochromic behavior of 3 are discussed with reference to experimental results, crystallographic data, and theoretical calculations.
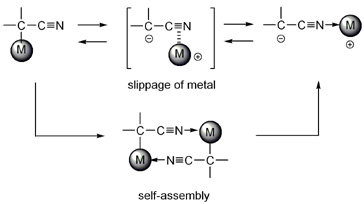
【Osaka University 100 Papers Selections】
Switchable C- and N-Bound Isomers of Transition Metal Cyanocarbanions: Synthesis and Interconversions of Cyclopentadienyl Ruthenium Complexes of Phenylsulfonylacetonitrile Anions,,
T. Naota, A Tannna, S. Kamuro, M. Hieda, K. Ogata, S-I. Murahashi, and H. Takaya, Chem. Eur. J., 14, 2482-2498 (2008).
The synthesis, structure, and dynamic behavior of bistable C- and N-bound isomers of transition-metal cyanocarbanions are described. A series of C-bound cyclopentadienyl (Cp) ruthenium phosphane complexes, [Ru{CH(CN)SO2Ph}(Cp)L1L2] (3), and their N-bound isomers, [Ru+(Cp)(NCCH-SO2Ph)L1L2] (4), were prepared by treating [RuCl(Cp)(PR3)2] with the sodium salt of phenylsulfonylacetonitrile and performing ligand-exchange reactions with the resulting compounds. Structural characterization by X-ray diffraction indicates that the cyanocarbanion moiety of 3 has an α-metalated structure, whereas that of 4 has a zwitterionic, end-on structure. Heating these complexes in aprotic solvents gives rise to irreversible linkage isomerization between C- and N-bound isomers, in which the relative thermal stabilities vary greatly depending on the steric and electronic nature of the ligands. Mechanistic studies of N-to-C isomerization revealed that the reaction proceeds irreversibly in a unimolecular manner without the formation of coordinatively unsaturated species. A metal-sliding process, which occurs over the -C-C≡N π-conjugated surface of the cyanocarbanion moiety, was suggested by results from kinetic studies and density functional theory (DFT) calculations. C-to-N isomerizations proceed by the above-mentioned intramolecular process, with a temperature-dependent contribution from the formation and cleavage of μ2-C,N coordination dimers [{Ru{CH(CN)SO2Ph}(Cp)(PPh3)}2] (15 and 16).
【Osaka University 100 Papers Selections】
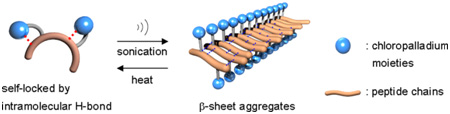
Ultrasound-Induced Gelation of Organic Fluids with Metallated Peptides,
K. Isozaki, H. Takaya, and T. Naota, Angew. Chem. Int. Ed., 46, 2855-2857 (2007).
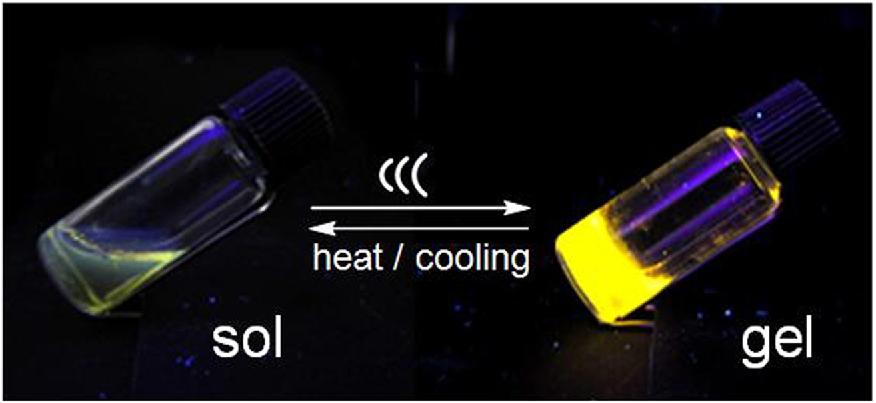
Stable solutions of a palladium-complexed dipeptide undergo gelation after brief ultrasound irradiation. This is the first case of a reversible, remotely controlled, and rapid sol?gel transition by H-bonding aggregates. By adjusting the sonication time, the gelation rates and heat-resistant properties of the aggregates can be controlled.
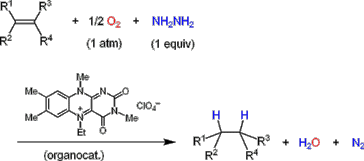
Flavin-Catalyzed Generation of Diimide: An Environmentally Friendly Method for the Aerobic Hydrogenation of Olefins,
Y. Imada, H. Iida and T. Naota, J. Am. Chem. Soc., 127, 14544-14545 (2005).
The first green and practical method for “aerobic hydrogenation” involving the use of hydrazine and an organocatalyst is described. Olefins can be hydrogenated by treatment with hydrazine in the presence of a 5-ethyl-3-methyllumiflavinium perchlorate (FlEt+・ClO4-) catalyst under O2 atmosphere to give the corresponding hydrogenated products in excellent yields along with environmentally benign water and molecular nitrogen as the only waste products.
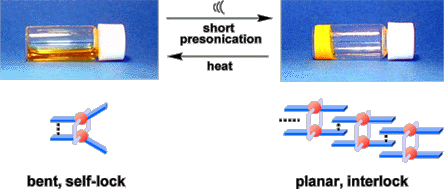
【Important Paper】【Osaka University 100 Papers Selections】
Molecules That Assemble by Sound: An Application to the Instant Gelation of Stable Organic Fluids,
T. Naota and H. Koori, J. Am. Chem. Soc., 127, 9324-9325 (2005).
The first molecule that assembles by ultrasound is described. An association-inert dinuclear Pd complex, anti-1a, which is stabilized by intramolecular π-stacking interactions, gelatinizes a variety of organic solvents instantly upon brief presonication for a few seconds. This is the first quick, positive, and reversible method for the remote switching of stable sol-gel phases. Uniquely, the rate can be precisely controlled over the range between “no gelation” and “instant gelation” simply by tuning the sonication time.
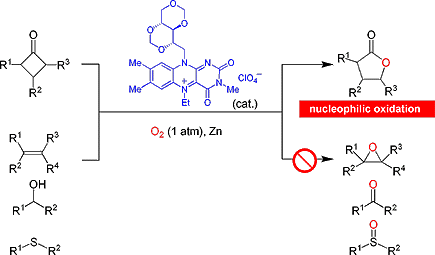
【Osaka University 100 Papers Selections】
An Aerobic, Organocatalytic, and Chemoselective Method for Baeyer-Villiger Oxidation,
Y. Imada, H. Iida, S.-I. Murahashi, and T. Naota, Angew. Chem. Int. Ed., 44, 1704-1706 (2005).
Highly chemoselective Baeyer?Villiger oxidations can be performed in the presence of other reactive functionalities such as alcohols, olefins, and sulfides, which would undergo electrophilic oxidation under conventional conditions (see scheme). [DMRFlEt]+[ClO4]? (depicted blue) is a new class of flavin compound that catalyzes aerobic Baeyer?Villiger oxidations in the presence of Zn dust as the electron source.
【Important Paper】【Osaka University 100 Papers Selections】
Flavin Catalyzed Oxidations of Sulfides and Amines with Molecular Oxygen,
Y. Imada, H. Iida, S. Ono, and S.-I. Murahashi, J. Am. Chem. Soc., 125, 2868-2869 (2003).
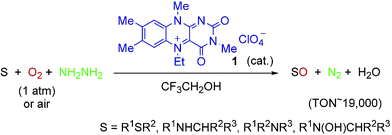
Novel biomimetic, aerobic oxidation with an organocatalyst was performed. The oxidations of organic substrates such as sulfides, secondary amines, N-hydroxylamines, and tertiary amines with molecular oxygen (1 atm) or even in air in the presence of 5-ethyl-3-methyllumiflavinium perchlorate catalyst and hydrazine monohydrate in 2,2,2-trifluoroethanol occur highly efficiently to give the corresponding oxidized compounds in excellent yields along with water and molecular nitrogen, which are environmentally benign. The TON of the oxidation of sulfides amounts to 19?000.
【Osaka University 100 Papers Selections】
Mechanism of the Interconversions between C- and N-Bound Transition Metal α-Cyanocarbanions,
T. Naota, A. Tannna, S. Kamuro, and S.-I. Murahashi, J. Am. Chem. Soc., 124, 6842-6843 (2002).
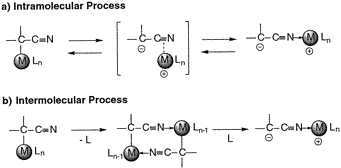
The first presentation of the intra- and intermolecular mechanisms of the C-N interconversions of transition metal α-cyanocarbanions is described. A pair of N- and C-bound isomers of isonitrile complex Ru+Cp(NCCH-SO2Ph)(PPh3)(CN-t-Bu) (1) and RuCp[CH(CN)SO2Ph](PPh3)(CN-t-Bu) (2) was synthesized for the mechanistic studies on the N-to-C isomerizations. Structural characterization by X-ray diffractions of 1 and 2 indicated their typical zwitterionic and α-metalated structures. The kinetic studies on the irreversible isomerization of 1 to 2 in benzene-d6 at 333−348 K were carried out using 1H NMR spectroscopy, affording the first-order rate constants k1 and the activation parameters ΔH‡ = 107±2 kJ·mol-1 and ΔS‡ = −22±5 J·K-1·mol-1. The almost identical values of k1 were obtained upon similar treatment of 1 with 4 equiv of external ligands such as PPh3, CH3CN, and t-BuNC at 333 K, indicating that the N-to-C isomerization proceeds in an intramolecular manner without dissociation of a ligand. As a model system for the C-to-N isomerization, the irreversible transformation of RuCp[CH(CN)SO2Ph](PPh3)2 (3) to Ru+Cp(NCCH-SO2Ph)(PPh3)2 (4) was investigated under various reaction conditions. The reaction of 3 at room temperature in THF affords the coordination dimers (RRu*,SC*,RRu*,SC*)-{RuCp[CH(CN)SO2Ph](PPh3)}2 (5) stereoselectively, and its distorted μ2-C,N-bound structure was determined by X-ray analysis. The reaction profiles for the isomerization of 3 includes the generation- and temperature-dependent decays of dimeric species 5 and its diastereomer 6, which strongly suggests that the intra- and intermolecular pathways are included in the C-to-N isomerization. The intramolecular process of the C-to-N isomerization of 3 has been confirmed by the kinetic studies on the isomerization of 3 with excess amount of PPh3 in benzene-d6 at 333−348 K which afford the first-order kinetics with the activation parameters of ΔH‡ = 121±1 kJ·mol-1 and ΔS‡ = 42±4 J·K-1·mol-1. Treatment of 5 with PPh3 in boiling benzene gives rise to the quantitative formation of N-bound complex 4. The controlled kinetic experiments on the cleavage of 5 with PPh3 have concluded that the cleavage of 5 with PPh3 proceeds via simultaneous C−Ru and N−Ru bond scissions, indicating the temperature-dependent participation of intermolecular process in the C-to-N isomerization of 3.
【Osaka University 100 Papers Selections】
Enantioselective Addition of Ketene Silyl Acetals to Nitrones Catalyzed by Chiral Titanium Complexes. Synthesis of Optically Active β-Amino Acids,
S.-I. Murahashi, Y. Imada, T. Kawakami, K. Harada, Y. Yonemushi, and N. Tomita, J. Am. Chem. Soc., 124, 2888-2889 (2002).
A chiral titanium complex, Ti(O-i-Pr)4 /BINOL/ tert-butylcatechol, catalyzes enantioselective addition reaction of ketene silyl acetals to nitrones to give optically active β-amino acid derivatives which are biologically active compounds and useful synthetic intermediates of natural products and pharmaceuticals such as β-lactam antibiotics. The combined process of catalytic oxidation of secondary amines and enantioselective carbon?carbon bond formation of nitrones thus obtained with ketene silyl acetals provides a useful two-step method for the synthesis of optically active β-amino acid derivatives and related nitrogen compounds.
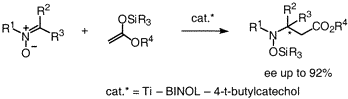
Asymmetric Baeyer-Villiger Reaction with Hydrogen Peroxide Catalyzed by a Novel Planar-Chiral Bisflavin,
S.-I. Murahashi, S. Ono and Y. Imada, Angew. Chem. Int. Ed., 41, 2366-2368 (2002).
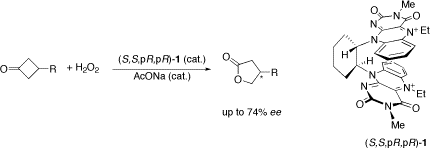
The chiral organocatalyst bisflavin 1 catalyzes the asymmetric Baeyer?Villiger reaction of cyclobutanones with H2O2 (see scheme). The corresponding lactones are obtained with up to 74?% ee.
【Important Paper】【Osaka University 100 Papers Selections】
Synthesis and Characterization of C- and N-Bound Isomers of Transition Metal α-Cyanocarbanions,
T. Naota, A. Tannna, S.-I. Murahashi, J. Am. Chem. Soc., 122, 2960-2961 (2000).




