HOME > Research
Research
The research in this group focuses on (1) design of new functional molecules for novel organic nanomaterials based on unrestricted and adventurous ideas, (2) synthesis of targeted molecules by exploring new reactions as well as methodologies founded by the power of modern organic synthesis, and (3) investigation on physical properties of the molecule in itself as well as formation and dynamics of the supramolecular structures by mean of advanced technologies for physical measurements. More specifically, we have been engaged in the following topics, which are detailed in the subsequent pages, to create organic molecules with new function and high performance for photonic and electronic applications: (i) synthesis of molecules having unique π-electronic systems with characteristic topology as well as giant conjugated π-electronic systems in which the size reaches nanometer scale, (ii) construction of nano-structures on solid surfaces by self-assembly of precisely designed molecules, and (iii) investigation of nano-meter scale molecular machines and switches by controlling various information programmed in the molecules to change in response to external stimuli.
Creation of Novel π-Electron Conjugated Compounds
 In order to exploit new substances with optoelectronic functions for prospective applications in future nanotechnology, we have been engaged in the synthesis and investigation of structurally novel π-electron conjugated compounds in which the size of the molecules reaches nanometer scale. In particular, we adhere to the creation of entirely new π-electron conjugated systems which have not been known. We therefore focus on the design and control of electronic states of two-dimensionally or three-dimensionally expanded conjugated systems consisting of sp- and sp2-hybridized carbons and their molecular shapes.
In order to exploit new substances with optoelectronic functions for prospective applications in future nanotechnology, we have been engaged in the synthesis and investigation of structurally novel π-electron conjugated compounds in which the size of the molecules reaches nanometer scale. In particular, we adhere to the creation of entirely new π-electron conjugated systems which have not been known. We therefore focus on the design and control of electronic states of two-dimensionally or three-dimensionally expanded conjugated systems consisting of sp- and sp2-hybridized carbons and their molecular shapes.
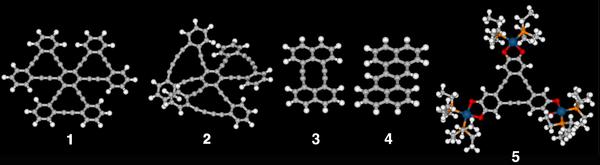
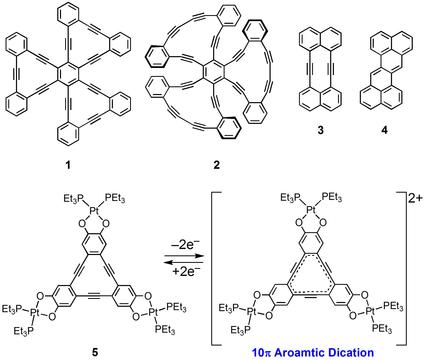
For instance, we synthesized trefoil-shaped molecule 1 consisting of three dehydrobenzo[12]annulene ([12]DBA) units, a substructure of hitherto unknown two-dimensional carbon network consisting of sp- and sp2-hybridized carbons, by exploring our original synthetic route. Due to efficient delocalization of π-electron in the planar framework, [12]DBA 1 shows a large two-photon adsorption cross section among hydrocarbons.
Compound 2 having three dehydrobenzo[14]annulenes ([14]DBAs) adopts a C3v symmetric, propeller-shaped nonplanar structure. Because compound 2 has a chiral propeller structure and relatively polar [14]DBA units, it is expected that compound 2 would show uni-directional rotation on a solid surface which can be controlled by external stimuli, an important function as a component of molecular scale motors.
Moreover, dehydronaphtho[10]annulene 3 was synthesized for the first time by our original synthetic method. We revealed that the transannular bond-forming reaction of 3 afforded derivatives of zethrene 4. Theoretical study predicts that zethrene possesses a singlet diradical character and exhibits large non-linear optical properties.
In addition, by incorporating of redox active metal catecholate units to the [12]DBA core, we established a novel and efficient method for oxidation of [12]DBA core. We revealed newly synthesized trinuclear platinum complex 5 was easily oxidized by both electrochemical and chemical methods, forming the corresponding stable dication which exhibited aromaticity due to the10π-electron conjugation.
Control of Two Dimensional Molecular Alignment on Solid Surfaces based on Precise Molecular Design
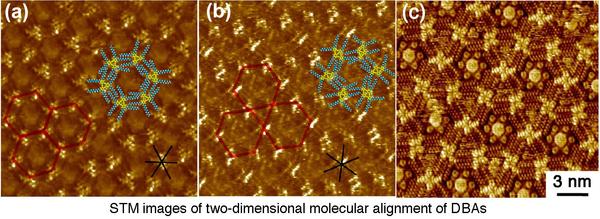
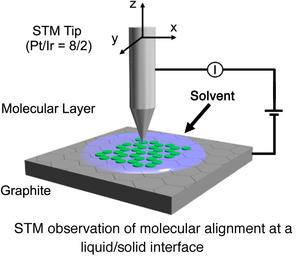 Recently, construction of two-dimensional (2D) molecular networks (or 2D crystals) on solid surfaces based on molecular self-assembly is a subject of intense interest because of the perspective for potential application in the field of nanoscience and nanotechnology. This technology would lead to the development of efficient and effective methods for surface patterning with a few nanometer-scale precisions. These 2D molecular networks are typically observed by means of scanning tunneling microscopy (STM) both under ultra high vacuum conditions and at liquid/solid interfaces (Right Figure).
Recently, construction of two-dimensional (2D) molecular networks (or 2D crystals) on solid surfaces based on molecular self-assembly is a subject of intense interest because of the perspective for potential application in the field of nanoscience and nanotechnology. This technology would lead to the development of efficient and effective methods for surface patterning with a few nanometer-scale precisions. These 2D molecular networks are typically observed by means of scanning tunneling microscopy (STM) both under ultra high vacuum conditions and at liquid/solid interfaces (Right Figure).
We have been engaging in the control of molecular alignment formed at the liquid/solid interface based on precisely designed organic molecules in which its self-assembling behaviors on surfaces are carefully programmed. To achieve precise control of molecular alignment and function on surfaces, high skills and deep knowledge in modern organic synthesis which allows creating designed molecular building blocks are necessary. Moreover, skills in STM manipulation are also required for successful and quick implementation of this research. By combining both technologies, we have been performing pioneering works in this area.
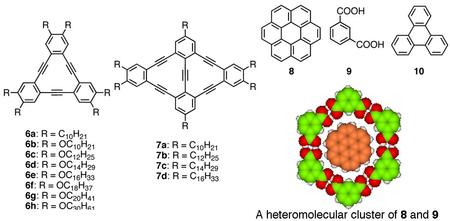
For example, decyl substituted triangular dehydrobenzo[12]annulene (DBA) 6a shows a honeycomb structure on a surface (Bottom Figure a), whereas rhombic-shaped fused DBA 7a with the same substituents displays a first Kagomé structure (Bottom Figure b). The 2D structural difference is arisen from the different core shape. Another intriguing feature of these networks is their intermolecular connectivity made of “alkyl chain interdigitation”. Triangular DBA with longer alkyl chains such as octadecyl group favorably form a non-porous linear pattern owing to its strong adsorbability. However, we found that the linear structure of the DBA transforms to a porous honeycomb structure upon dilution. This research presents the first demonstration of solute concentration dependency of the 2D network structure and thus becomes a general guideline for control of self-assembly at the liquid/solid interface.
To develop more complex and sophisticated 2D networks consisting of multiple components, we observed the formation of a three-component 2D crystal, which represents the world’s second example of such structure. Namely,
a heteromoelcular cluster consisting of coronene 8 and six molecules of isopthalic acid 9 adsorbs in the pore of the honeycomb network of DBA 6b. Furthermore, we succeeded in the construction of four-component structure for the first time using the Kagomé structure of rhombic bisDBA 7b following the same strategy (Bottom Figure c). A heteromolecular cluster of 8 and 9 and triphenylene 10 were adsorbed at the large hexagonal pore and small triangular pore of the Kagomé structure, respectively. Central to the construction of such multi-component networks are the combination of two intermolecular interactions (van der Waals and hydrogen bonding interactions) and size and shape complementarities between the guest molecule as well as the guest cluster and the respective pores.
Creation of Functional Molecules Based on Supramolecular Chemistry
Suprmolecular chemistry covers intermolecular interactions, design of molecular assemblies, and bestowal of functions to the assemblies. Therefore, supramolecular chemistry is important for sensing technology, separation technology, and bottom-up approach in nanoscience and nanotechnology.
Precise Molecular Recognition:
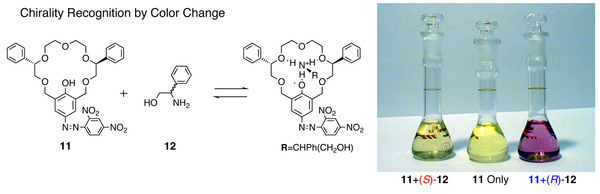
We are studying on design and functionalization of host molecules which form complexes selectively with amino compounds with multiple non-covalent interactions. We have developed optically active crown ethers that are capable of discriminating chirality of chiral amino compounds with extremely high enantiomer selectivity. We designed and synthesized crown ether 11 for optical chiral sensing of chiral amines (e.g. ethanol amine 12) by combining the crown ether unit with 2,4-dinitrophenylazo group. With this chiral sensor, chirality of the guest molecule was clearly differentiated by spectral changes of UV-visible absorption spectra and clear color change was realized as shown.
Crown ether 13 having a conjugated π-electron system (a molecular wire structure) demonstrated effective signal transduction and amplification. With this crown ether, chirality information of the guest amines is transduced into the difference of fluorescent intensity to a significant extent. In addition, we have developed chiral selector 14 containing the crown ether unit for chiral chromatography column, which represents the first commercially available selector covalently bound to the support (i.e. silica gel).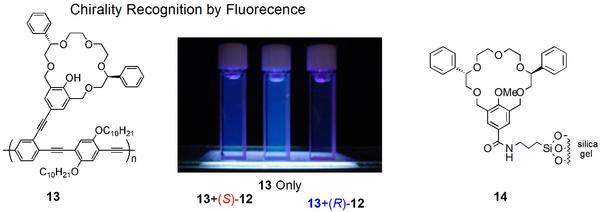
Molecular Machines:
We have synthesized a number of functional rotaxanes, which we expect to play a central role in the future development of intelligent molecular systems such as molecular switches, molecular brakes, and molecular motors. For example, in rotaxane 15 the ring component in a large size state (blue) shuttles back and forth quickly between two (red) stations of the axle component separated by the (yellow) spacer. When the size of the ring component is reduced (green) by external stimuli (e.g. photo irradiation), the rate of the shuttling motion of rotaxane 16 is reduced significantly. The small ring component of 16 converts back to the large ring state (i.e. 15) by thermal reaction. Thus the brake function for the shuttling motion is reversible. The actual molecules which realized the above molecular brake scenario are 17 and 18 composed of a photo and thermally reactive ring molecule having anthracene units and an axle molecule with two ammonium stations. The rate of shuttling motion of 17 was proved to be reduced to less than 1% through conversion to 18 by photo irradiation.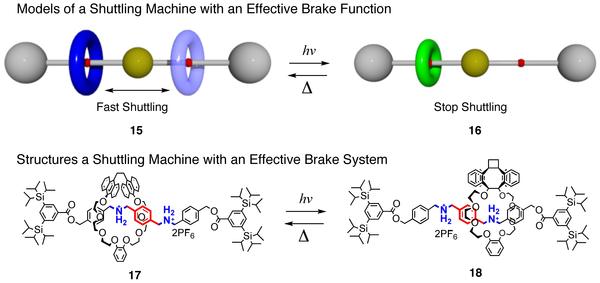 >>Click here to see 15 in motion
>>Click here to see 15 in motion