HOME > Research
Research
The research in this group focuses on "The Development of Synthetic Supramolecular Chemistry" which will supply novel materials having unique structures, dynamics, and functions. In order to created such materials, well-designed several molecules are synthesized and combined using intermolecular-interaction based on our skills and knowledges of synthetic organic chemistry to afford promising artificial supramolecules. The structures and the physical properties of the obtained artificial supramolecules are then determined and investigated using high level of facilities which will contribute to get important information of the unique structure and resulting specific properties of the supramolecules including dynamics of components for example. More concretely, creation of organic compounds having novel functions and excellent properties are carried out. In particular, molecular machines at nano-level responsible to external stimuli, precise molecular sensors based on well-designed molecular assemblies, and functional organic materials in general are now developing.
Synthesis and Properties of Suprammolecules having Mechanically Interlocked Structures
Compounds having mechanically interlocked ring components and dumbbell shaped axle components are called rotaxanes. One of the applications of rotaxane chemistry is to make smallest machines at molecular level. Nobel prize in chemistry was awarded to three researchers working in this field, J. -P. Sauvage, Sir J. F. Stoddart and B. L. Feringa in 2016. Rotaxane shown below is consist of ring components having Cs symmetry and dumbbell shaped axle having different stopper units at the terminals. The pair of rotaxanes shown are mirror images each other and non-superimposable. The rotaxanes are new materials having chirality specific to the rotaxane structure, which have been able to be synthesized by development of synthetic supramolecular chemistry. recently, these rotaxanes can be able to be synthesized with high selectivity and high chemical yield in our group. Moreover, isolation of the rotaxanes in an optically pure form. Concrete structure of the rotaxane is shown below. This material is thought to be act as chiral sensor and material for optical resolution etc promising from the view of application.
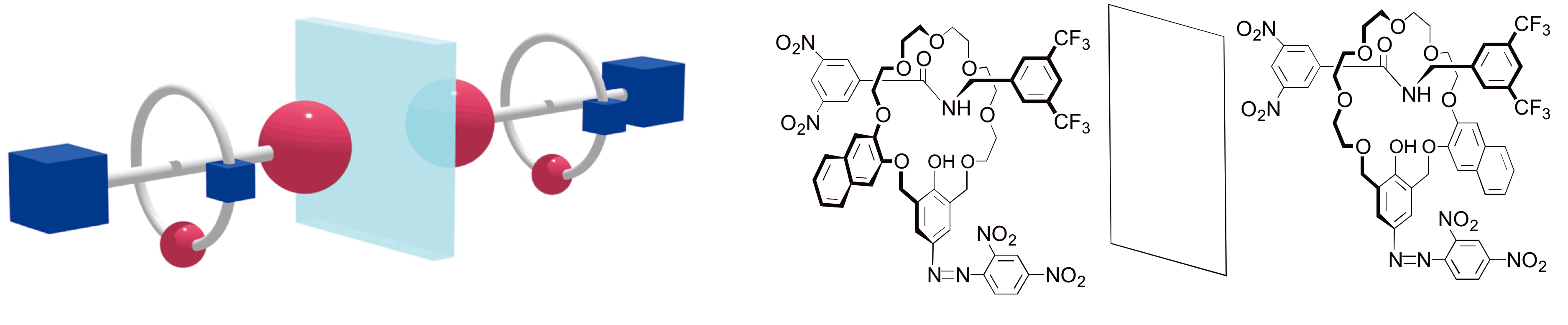
As an example of applications for chirality recognition sensor, this material is sued for chiral indicator. Chiral indicator compound (left), substrate amine RPGO (middle), and photo showing clear color change of the solution containing the indicator and substrate with different concentration. The indicator change color of the solution by trapping the substrate RPGO very clearly.

Development of Molecular Machines and Molecular Switches Responsible to External Stimuli
Supramolecular chemistry is chemistry beyond molecules, which deals design, assemble, and organization of molecules. Supramolecular chemistry is fundamental for bottom-up type Nano technology and science. Supramolecular chemistry treat intermolecular interaction to design molecular aggregation and function of the aggregates.
We are investigating developments of functional supramolecules such as molecular switches, molecular brakes, molecular motors based on molecular recognition phenomena. Especially development of synthetic method and control of motions of rotaxanes having unique dynamics by external stimuli like photo and heat irradiation.
As an example, ring component of the rotaxane shown below are shuttling between two ammonium stations in the axle component. By photo irradiation the size of the ring shrinks and the shuttling motion becomes slow, Brake ON! The ring return to the original size by heating, therefore this brake system is reversible. The brake is supramolecule consists of photo and heat responsible crown ether and ammonium axle as shown below. Reversible and quantitative brake was realized using light and heat. It was revealed that the shuttling velocity was reduced less than 1% in this system.
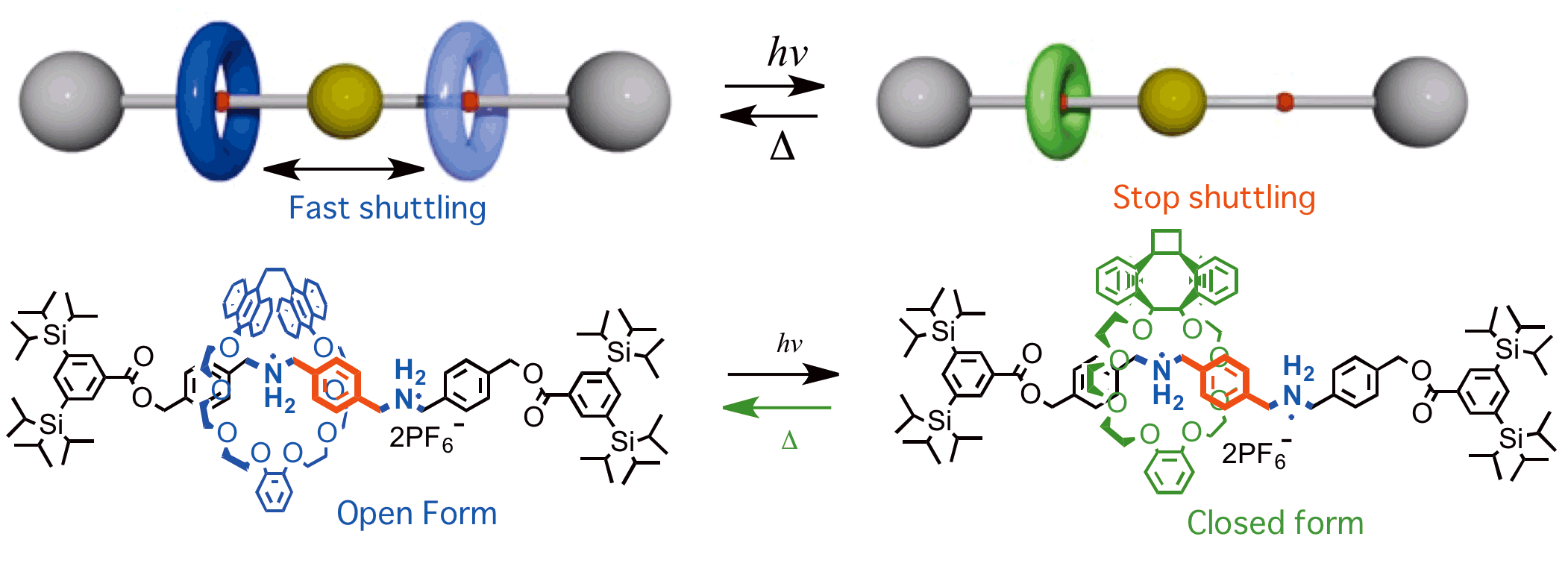 >>Click here to see shuttling in motion
>>Click here to see shuttling in motion
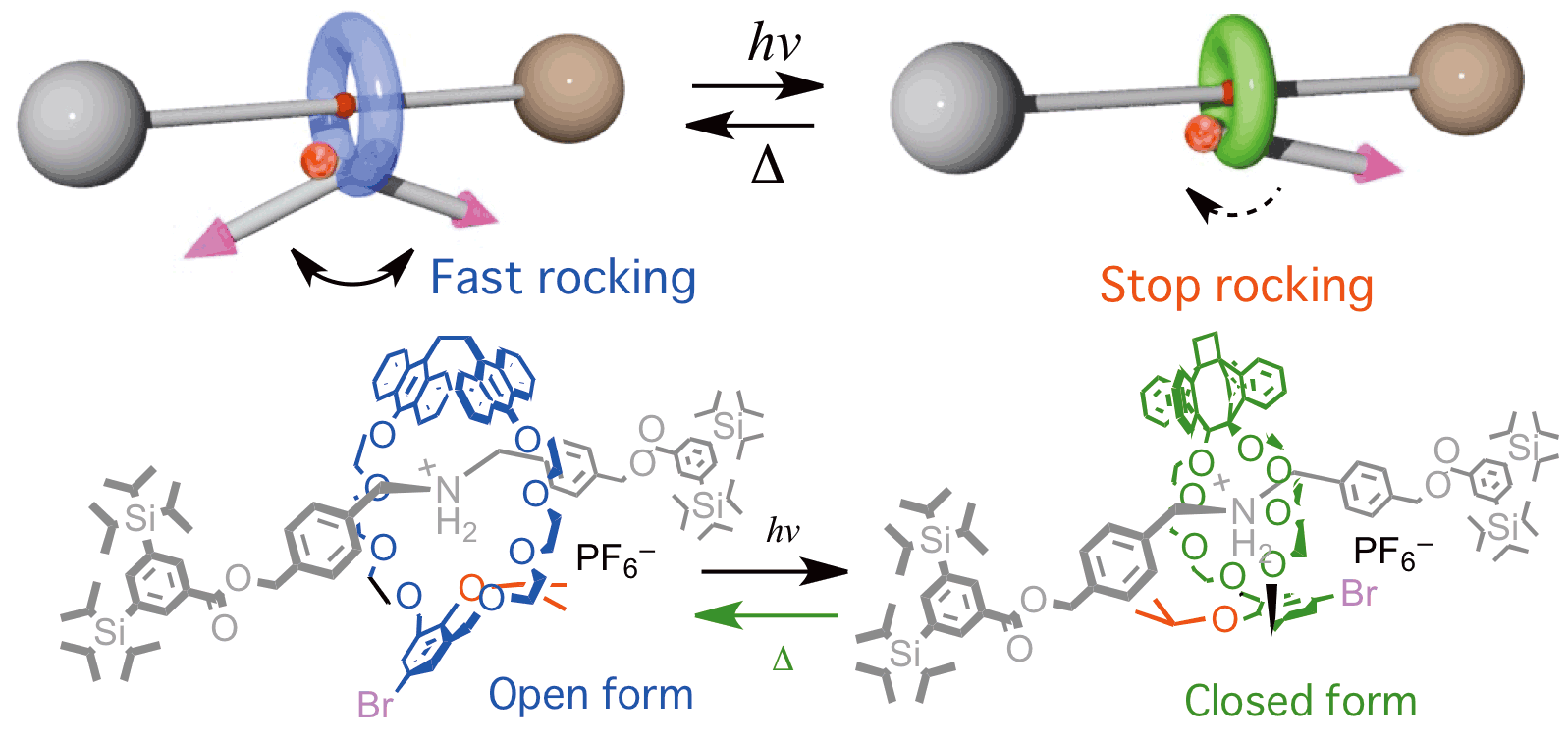
Rotaxane shown below is one of unique molecular brakes which reduce rocking motion of pendulum moiety (shown as arrow). The rocking velocity was revealed to be reduced less than 0.05%.
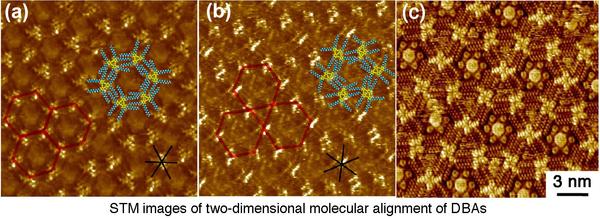
Control of Two Dimensional Molecular Alignment on Solid Surfaces based on Precise Molecular Design
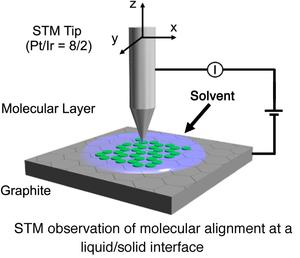 Recently, construction of two-dimensional (2D) molecular networks (or 2D crystals) on solid surfaces based on molecular self-assembly is a subject of intense interest because of the perspective for potential application in the field of nanoscience and nanotechnology. This technology would lead to the development of efficient and effective methods for surface patterning with a few nanometer-scale precisions. These 2D molecular networks are typically observed by means of scanning tunneling microscopy (STM) both under ultra high vacuum conditions and at liquid/solid interfaces (Right Figure).
Recently, construction of two-dimensional (2D) molecular networks (or 2D crystals) on solid surfaces based on molecular self-assembly is a subject of intense interest because of the perspective for potential application in the field of nanoscience and nanotechnology. This technology would lead to the development of efficient and effective methods for surface patterning with a few nanometer-scale precisions. These 2D molecular networks are typically observed by means of scanning tunneling microscopy (STM) both under ultra high vacuum conditions and at liquid/solid interfaces (Right Figure).
We have been engaging in the control of molecular alignment formed at the liquid/solid interface based on precisely designed organic molecules in which its self-assembling behaviors on surfaces are carefully programmed. To achieve precise control of molecular alignment and function on surfaces, high skills and deep knowledge in modern organic synthesis which allows creating designed molecular building blocks are necessary. Moreover, skills in STM manipulation are also required for successful and quick implementation of this research. By combining both technologies, we have been performing pioneering works in this area.
For example, decyl substituted triangular dehydrobenzo[12]annulene (DBA) 6a shows a honeycomb structure on a surface (Bottom Figure a), whereas rhombic-shaped fused DBA 7a with the same substituents displays a first Kagomé structure (Bottom Figure b). The 2D structural difference is arisen from the different core shape. Another intriguing feature of these networks is their intermolecular connectivity made of "alkyl chain interdigitation". Triangular DBA with longer alkyl chains such as octadecyl group favorably form a non-porous linear pattern owing to its strong adsorbability. However, we found that the linear structure of the DBA transforms to a porous honeycomb structure upon dilution. This research presents the first demonstration of solute concentration dependency of the 2D network structure and thus becomes a general guideline for control of self-assembly at the liquid/solid interface.
To develop more complex and sophisticated 2D networks consisting of multiple components, we observed the formation of a three-component 2D crystal, which represents the world's second example of such structure. Namely,
a heteromoelcular cluster consisting of coronene 8 and six molecules of isopthalic acid 9 adsorbs in the pore of the honeycomb network of DBA 6b. Furthermore, we succeeded in the construction of four-component structure for the first time using the Kagomé structure of rhombic bisDBA 7b following the same strategy (Bottom Figure c). A heteromolecular cluster of 8 and 9 and triphenylene 10 were adsorbed at the large hexagonal pore and small triangular pore of the Kagomé structure, respectively. Central to the construction of such multi-component networks are the combination of two intermolecular interactions (van der Waals and hydrogen bonding interactions) and size and shape complementarities between the guest molecule as well as the guest cluster and the respective pores.
Access / Contact
Aoociate Professor Keiji Hirose 1-3 Machikaneyama-cho, Toyonaka 560-8531 Osaka, JapanPhone:+81-(0)6-6850-6225
FAX:+81-(0)6-6850-6229
E-mail:
hirose#chem.es.osaka-u.ac.jp (Please replace # mark in e-mail address to @ mark.)
[Access Map]
Toyonaka Campus