Research
Our modern life cannot exist without organic compounds. They are widely utilized in our daily life ranging from medicines, fibers, and plastics. Because organic compounds can display various functions depending on their molecular structures, it is highly important to develop synthetic methods to produce organic compounds with precisely controlled structures at a molecular level.
In the Shintani research group, we are actively involved in multiple areas of research, particularly focusing on the following three aspects:
- “Design Reactions: development of new selective organic reactions”
- “Design Molecules: creation of new organic molecules;
- “Design Functions: exploration of new functional organic compounds”
1. Stitching Reaction toward New Functional π-Conjugated Compounds
Silicon-bridged π-conjugated compounds are widely investigated as potentially applicable functional organic materials based on their optoelectronic properties. However, the accessible molecular structures are currently limited with existing synthetic methods. It is therefore necessary to develop new methods that allow for the synthesis of a wide variety of silicon-bridged π-conjugated compounds in order to realize new functional organic compounds. In this regard, our group is trying to synthesize silicon-bridged π-conjugated compounds possessing novel molecular skeletons through the development of new synthetic strategies.
As a new synthetic strategy, we recently developed a “stitching reaction” that can rapidly provide a bridged π-conjugated compound through the formation of multiple carbon–carbon bonds between two linear substrates in a stitching manner by the action of a transition metal catalyst. So far, we have successfully synthesized silicon-bridged ladder-type π-conjugated compounds with up to six silicon units [1,2]. These compounds are highly air-stable even with extended π-conjugation and they can also function as organic elements for single molecular transistors, which are expected to contribute to the progress of nanotechnology.
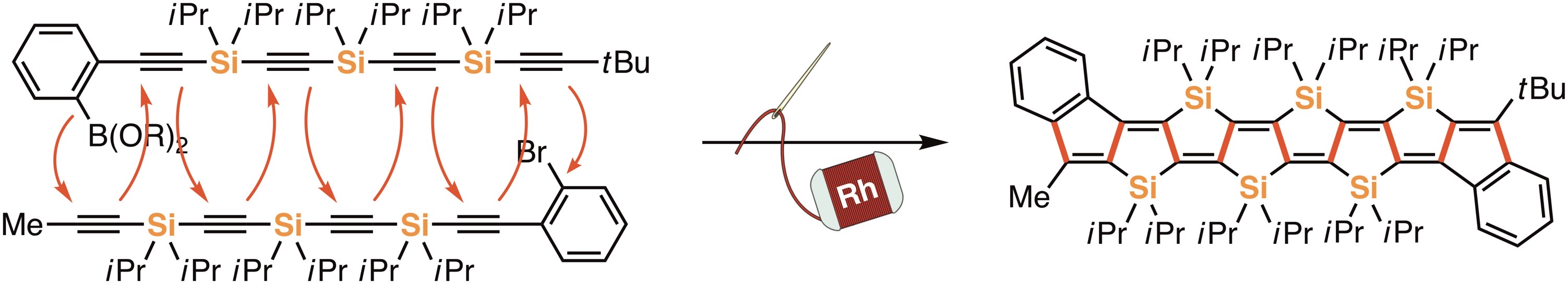
Recent publication:
- Shintani, R.; Iino, R.; Nozaki, K. J. Am. Chem. Soc. 2016, 138, 3635.
- Shintani, R.; Misawa, N.; Tsuda, T.; Iino, R.; Fujii, M.; Yamashita, K.; Nozaki, K. J. Am. Chem. Soc. 2017, 139, 3861.
- Lee, S. J.; Kim, J.; Tsuda, T.; Takano, R.; Shintani, R.; Nozaki, K.; Majima, Y. Appl. Phys. Express 2019, 12, 125007.
2. New Molecular Transformations toward Functional Organosilicon Compounds
Silicon-containing organic compounds, organosilanes, do not exist in nature, but they constitute an attractive class of compounds that can be used as biologically active substances or functional organic materials, in addition to intermediates for organic synthesis. However, accessible molecular structures are currently limited, it is therefore necessary to develop new molecular transformations for further expansion of functional organosilanes. Our group is particularly focusing on the creation of various organosilicon compounds that are difficult to synthesize by rapidly constructing complex molecular skeletons via selective functionalization of unreactive C–H bonds under appropriate transition-metal catalysis. We are also engaged in the discovery of new molecular functions through investigations of their optical and electronic properties.
For example, we recently achieved an efficient synthesis of light-emitting benzophenanthrosilines from readily accessible alkyne-containing aryl triflates under simple palladium catalysis [1]. In addition, similar starting compounds can provide benzofluorenosilepins, a new class of π-conjugated organosilanes, under slightly modified conditions through a different reaction pathway [2]. We are also involved in the elucidation of the reaction mechanisms of these catalytic reactions and further creation of useful organic compounds by the development of new transformations based on the mechanistic insights.
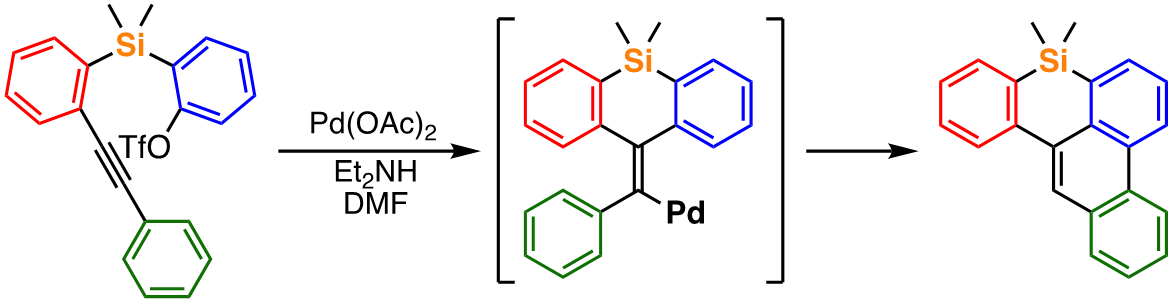
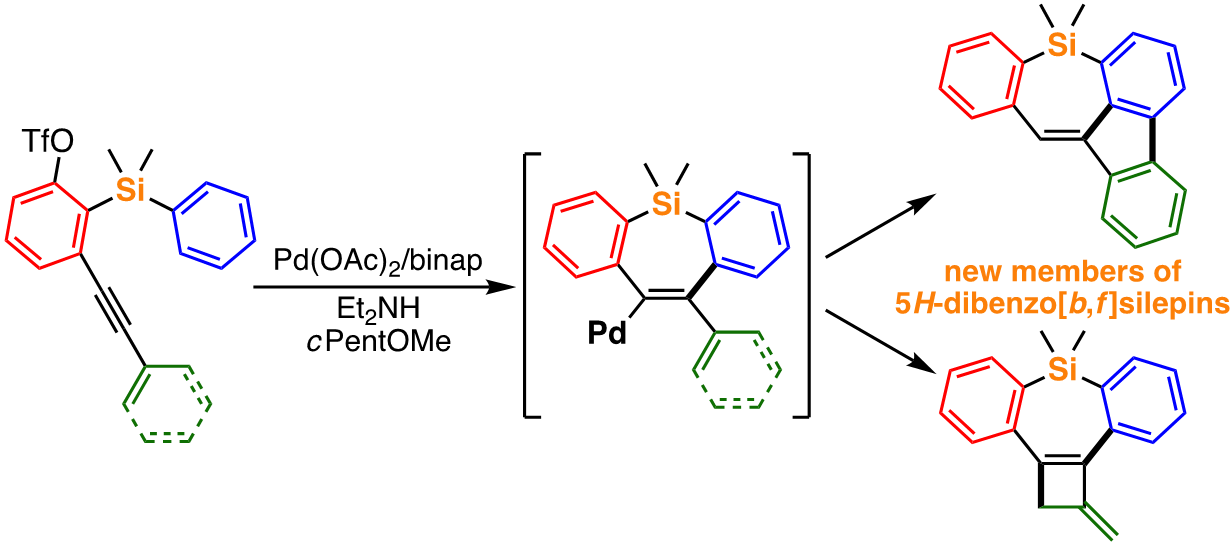
Recent publication:
- Tsuda, T.; Kawakami, Y.; Choi, S.-M.; Shintani, R. Angew. Chem., Int. Ed. 2020, 59, 8057.
- Tsuda, T.; Choi, S.-M.; Shintani, R. J. Am. Chem. Soc. 2021, 143, 1641.
3. New Polymerization Reactions toward New Polymer Synthesis
Transition-metal-catalyzed reactions are indispensable in modern synthetic organic chemistry, and this is also true for the polymer synthesis. Various interesting reactivity has been found for small molecule synthesis depending on the choice of catalyst metals and ligands. If this knowledge can be effectively applied to polymer synthesis, efficient synthesis of new functional polymers that are currently inaccessible may become possible. In this regard, our group aims to develop new types of polymerization reactions by exploiting the characteristics of transition-metal catalysts.
For instance, catalytic reactions involving insertion of a carbon–carbon unsaturated bond into an organorhodium species are widely utilized as one of the powerful synthetic methods. In addition to this insertion process, organorhodium species are also known to undergo intramolecular 1,4-migration to generate different (isomeric) organorhodium species, and several synthetic reactions involving this process have been developed. However, this characteristic feature of organorhodium species had not been applied to the polymer synthesis. In this context, we recently developed a new type of rhodium-catalyzed polymerization reaction consisting of alkene insertion/1,4-rhodium migration, and successfully synthesized a new polymer, poly(cyclopropylene–o-phenylene), by employing 3,3-diarylcyclopropene as monomer [1]. In addition, through the development of a new mode of polymerization “stitching polymerization”, we achieved efficient synthesis of new π-conjugated polymers that are difficult to synthesize by conventional polymerization methods and evaluated the physical properties of the obtained polymers as well [2,3].
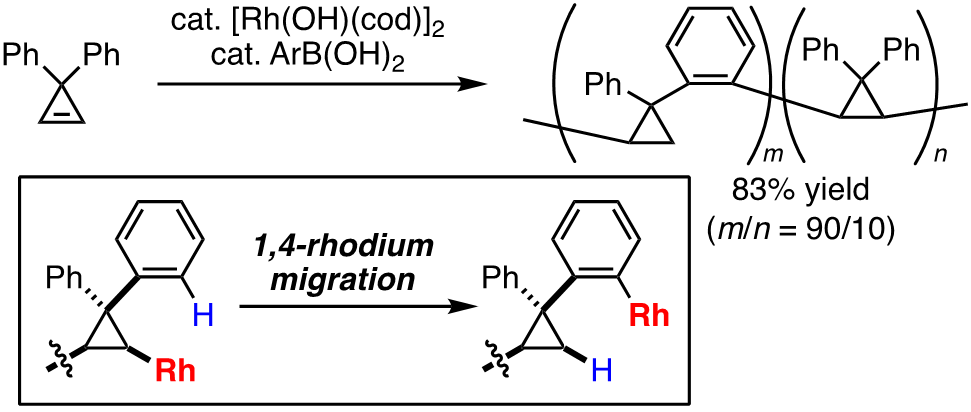
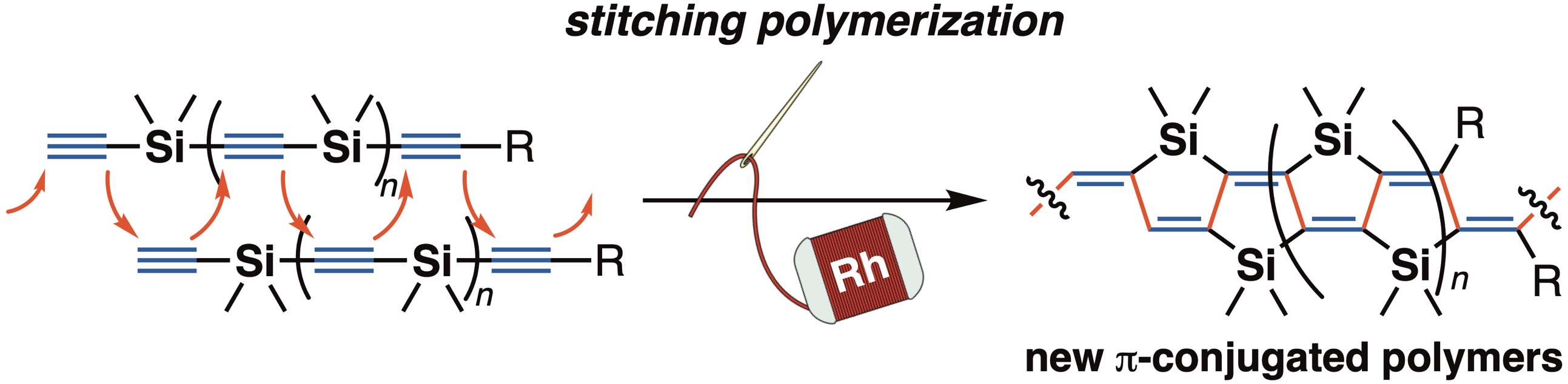
Recent publication:
- Shintani, R.; Iino, R.; Nozaki, K. J. Am. Chem. Soc. 2014, 136, 7849.
- Ikeda, S.; Shintani, R. Angew. Chem., Int. Ed. 2019, 58, 5734.
- Ikeda, S.; Hanamura, Y.; Tada, H.; Shintani, R. J. Am. Chem. Soc. 2021, 143, 19559.
4. Development of π-Conjugated Zwitterions with Open-Shell Character
π-Conjugated molecules with open-shell character have been actively investigated in recent years not only because of their properties such as near-infrared (NIR) absorption and amphoteric redox properties originating from their small HOMO–LUMO energy gaps but also their unique optical and magnetic properties originating from the open-shell electronic structures, which are not observed for closed-shell π-conjugated molecules. Most open-shell singlet biradicals isolated to date are fused π-conjugated molecules with o- or p-quinodimethane moieties, which are described by quinoid and singlet biradical canonical structures. On the other hand, only a few studies have been made on π-conjugated zwitterions with open-shell character, which are described by zwitterion and singlet biradical canonical structures. This deficiency hampers detailed understanding of the electronic structures and properties of π-conjugated zwitterions with open-shell character. In this context, our group is actively investigating in this underexplored research area.
For instance, 13b-azoniadibenzo[a,j]phenalen-5-ide, which was designed and synthesized by our group, has no quinoid structure, and is described by resonance structures consisting of zwitterionic structures I and II and singlet biradical structure III. This zwitterion is promising as a basic structure for functional π-conjugated molecules because of its NIR absorption (ca. 1100 nm) and amphoteric redox properties (ΔEredox = 1.48 eV) originating from small HOMO–LUMO energy gap. Its open-shell character was confirmed by observation of the thermally-excited triplet state together with DFT calculations [1].
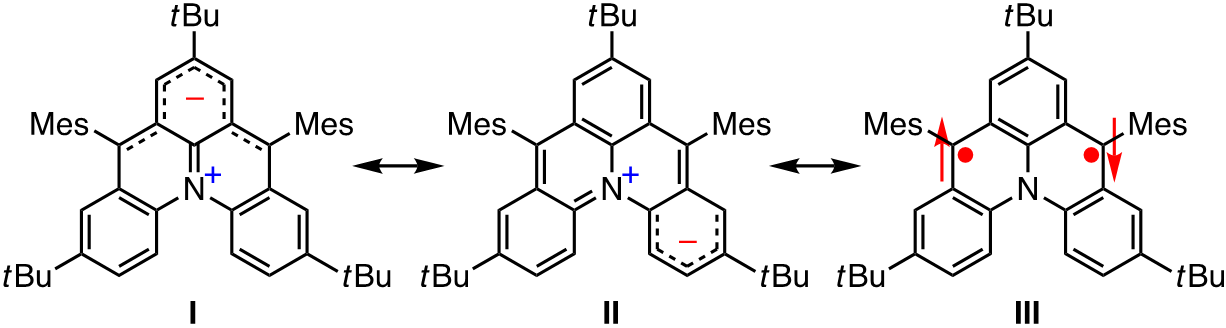
Recent publication:
- Arikawa, S.; Shimizu, A.; Shintani, R. Angew. Chem., Int. Ed. 2019, 58, 6415.
5. Synthesis and Properties of Hydrocarbons with a Triplet Ground State
Molecules with a triplet ground state, in which two unpaired electrons ferromagnetically interact, can be the basic motif of magnetic materials. Various organic molecules with a triplet ground state based on stable radicals such as nitroxide have been synthesized and their magnetic properties have been elucidated. On the other hand, hydrocarbons with a triplet ground state are highly reactive, which limited their studies mostly to generation and observation in low-temperature matrices, and their crystal structures remained elusive. In this context, our group is actively studying to synthesize and isolate hydrocarbons with a triplet ground state and elucidate their magnetic, optical, and electrochemical properties.
For instance, triangulene, known as Clar’s hydrocarbon, has been the target of synthesis for many research groups since 1950s. However, triangulene as well as its derivatives have not been successfully isolated. We designed a triangulene derivative having bulky substituents at its appropriate positions and succeeded in its synthesis and isolation [1]. The crystal structure was also successfully obtained to confirm its high symmetry. The triangulene derivative was confirmed to have a triplet ground state with significant interaction between unpaired electrons and it exhibited a reversible two-electron oxidation wave to the dication and a reversible two-electron reduction wave to the dianion by electrochemical analysis.
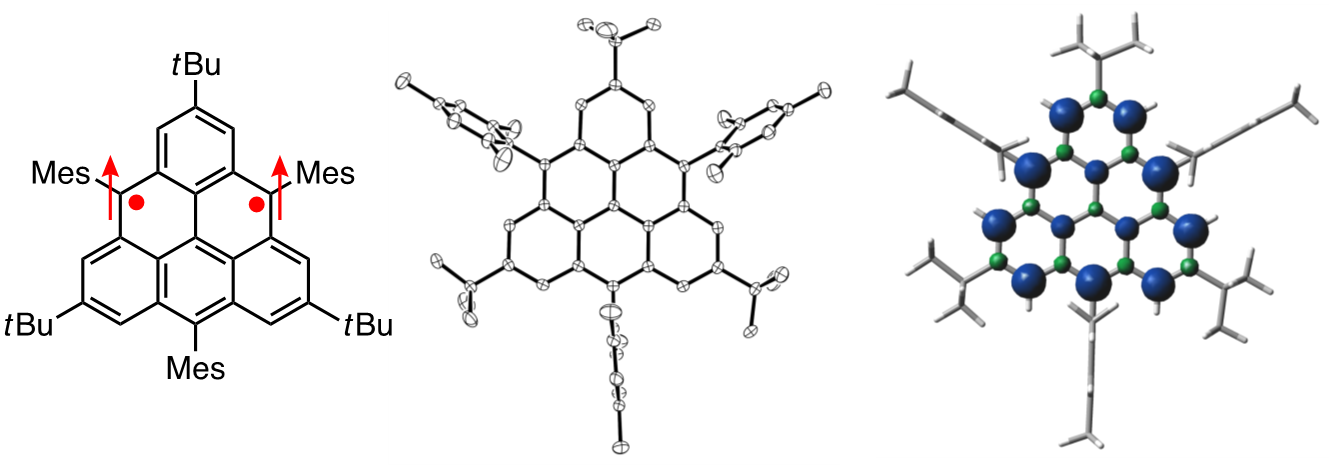
Recent publication:
- Arikawa, S.; Shimizu, A.; Shiomi, D.; Sato, K.; Shintani, R. J. Am. Chem. Soc. 2021, 143, 19599.
